Alpha Synuclein: Synuclein Family
Potential Targets in the Synuclein Family
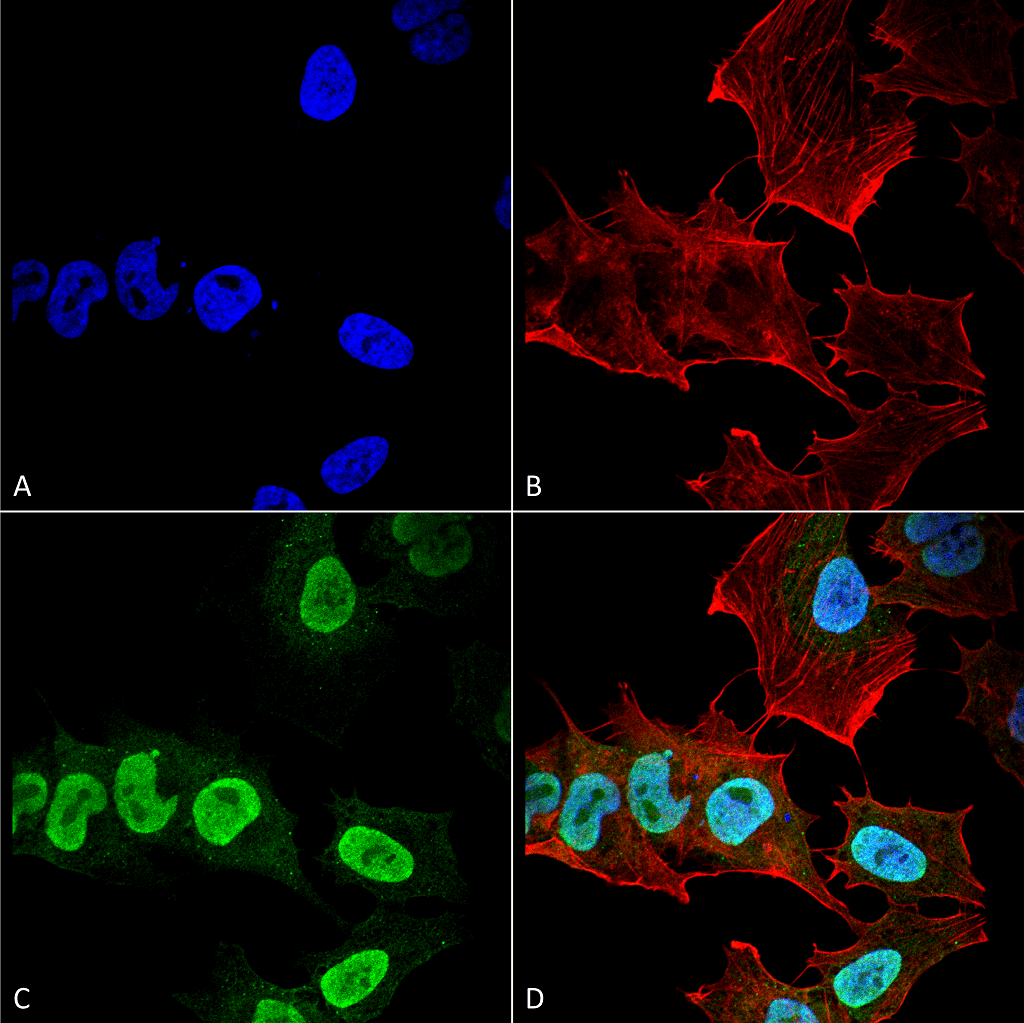
The synuclein family consists of three small, soluble proteins; alpha, beta, and gamma synuclein. Each contains a conserved alpha-helical lipid-binding motif and is expressed in neural tissue and certain tumors of vertebrates. Alpha and beta are found mainly in the brain, while gamma is found in the peripheral nervous system. The normal functions of these proteins are unknown; however, some hypothesize that they mediate membrane stability and turnover39.
Mutations in alpha synuclein have been heavily associated with Parkinson’s disease as the protein constitutes LBs and LNs. The beta and gamma synuclein are not located in LBs or LNs, but they are linked to hippocampal axon pathology in Parkinson’s disease40. Therefore, beta and gamma synuclein, may be potential targets in PD therapy.
Beta synuclein shares 60% sequence identity with alpha synuclein, however, it is not currently a primary target in PD therapy. Beta synuclein is fibril resistant under normal cytoplasmic conditions, however, the protein was reported to undergo a toxic gain of function mutation in response to changes in cytosolic pH and produce beta synuclein fibrils. At a pH of 5.8, beta synuclein fibrilization begins to occur41, which opens up the possibility of targeting beta synuclein in PD treatment.
The synucleins, additionally, seem to work together to uphold the PD phenotype42. Alpha synuclein is known to regulate dopamine, therefore knocking out this protein should lead to higher dopamine levels in the cell. Yet, mice missing any one synuclein protein fail to exhibit improved dopamine levels and a change in the disease phenotype, perhaps due to the other synuclein proteins pathogenically compensating for the absence. Therefore, knocking out or inhibiting beta and gamma synuclein as well as alpha synuclein, could be a method of restoring dopamine levels in PD treatment.