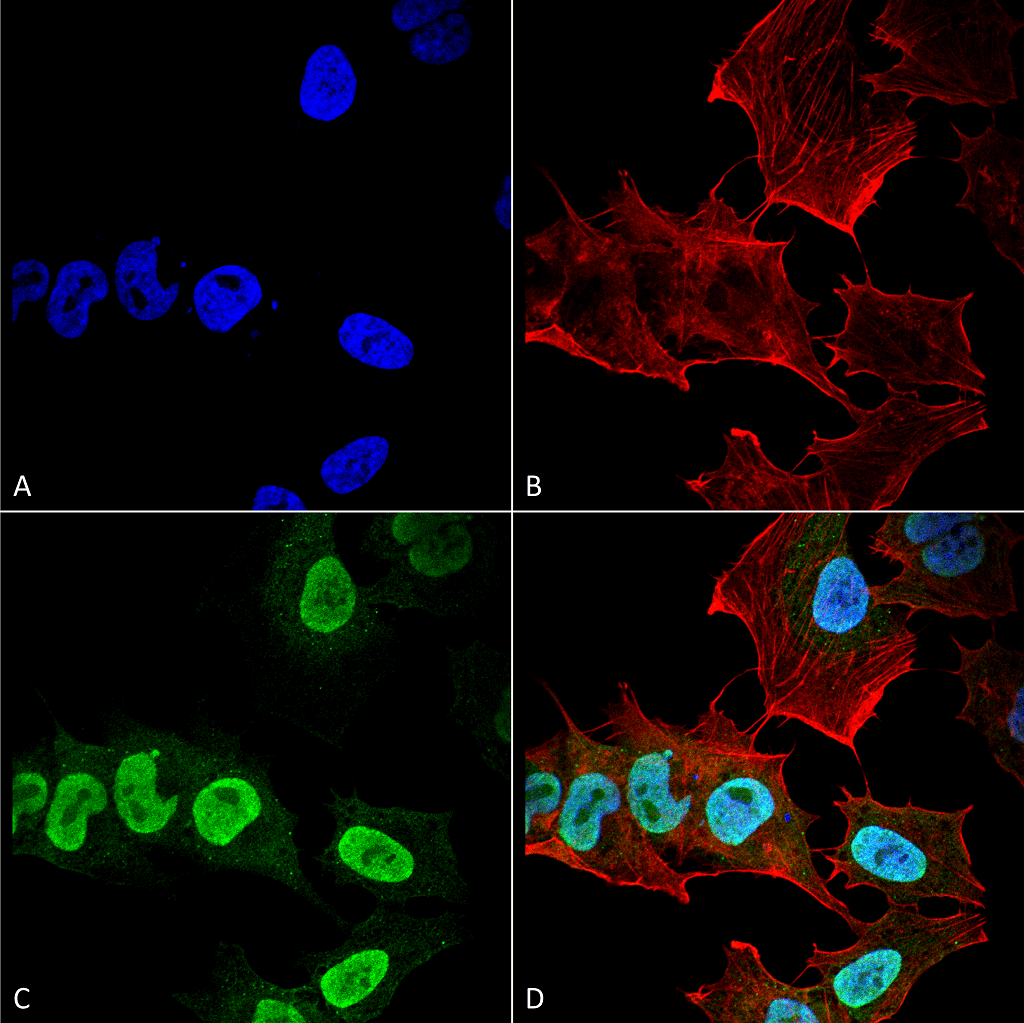
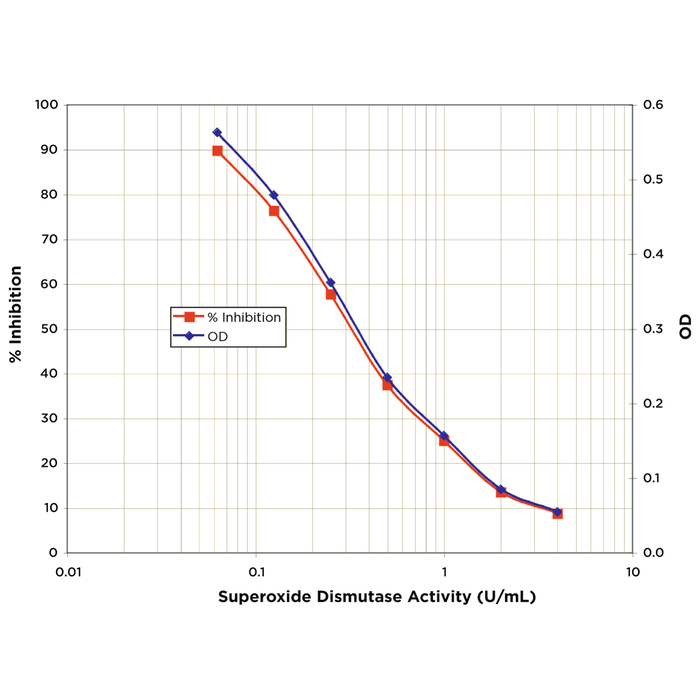
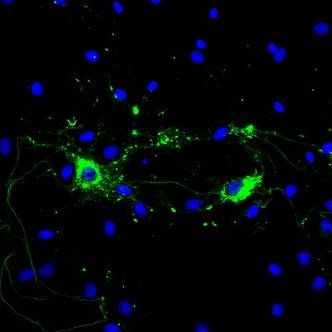
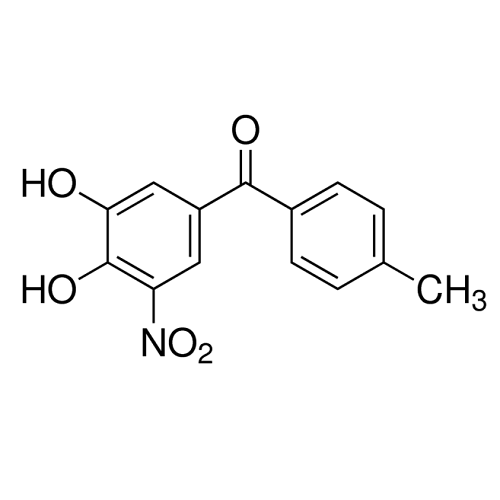
Alpha Synuclein: Structure
Alpha synuclein is a 140-amino acid protein that contains a sequence motif unique to the synuclein family10. The protein has three different domains in its structure: 1) an N- terminal (residues 1-60), 2) a central NAC domain (residues 61-95), and 3) a C- terminal (96-140). The N- terminal region contains all the mutations associated with PD, and consists of conserved residue repeats (KTK(E/Q)GV) that form amphipathic alpha helixes. The size of the repeats, along with their lipid binding ability enables the peptide to form three helical turns and directly contact the membrane. The polar C- terminus is defined by an abundance of three residues (proline, aspartate, and glutamate)3 and is less conserved than the N- terminus11. A C- terminal deletion causes increased alpha synuclein aggregation, which suggests that this domain regulates protein accumulation10. Finally, the NAC domain contains many hydrophobic amino acids that are key in alpha synuclein aggregation3.
There is conflicting research on alpha synuclein’s native structure; originally the protein was described as unfolded, but recent discoveries have suggested that it remains folded in a solution. For example, Wang et al. (2011) described alpha synuclein as a stable helically folded tetramer without lipid bilayers or micelles. The most plausible explanation for the findings of multiple conformations of alpha synuclein is that the protein exists in many oligomeric states in a dynamic equilibrium3.