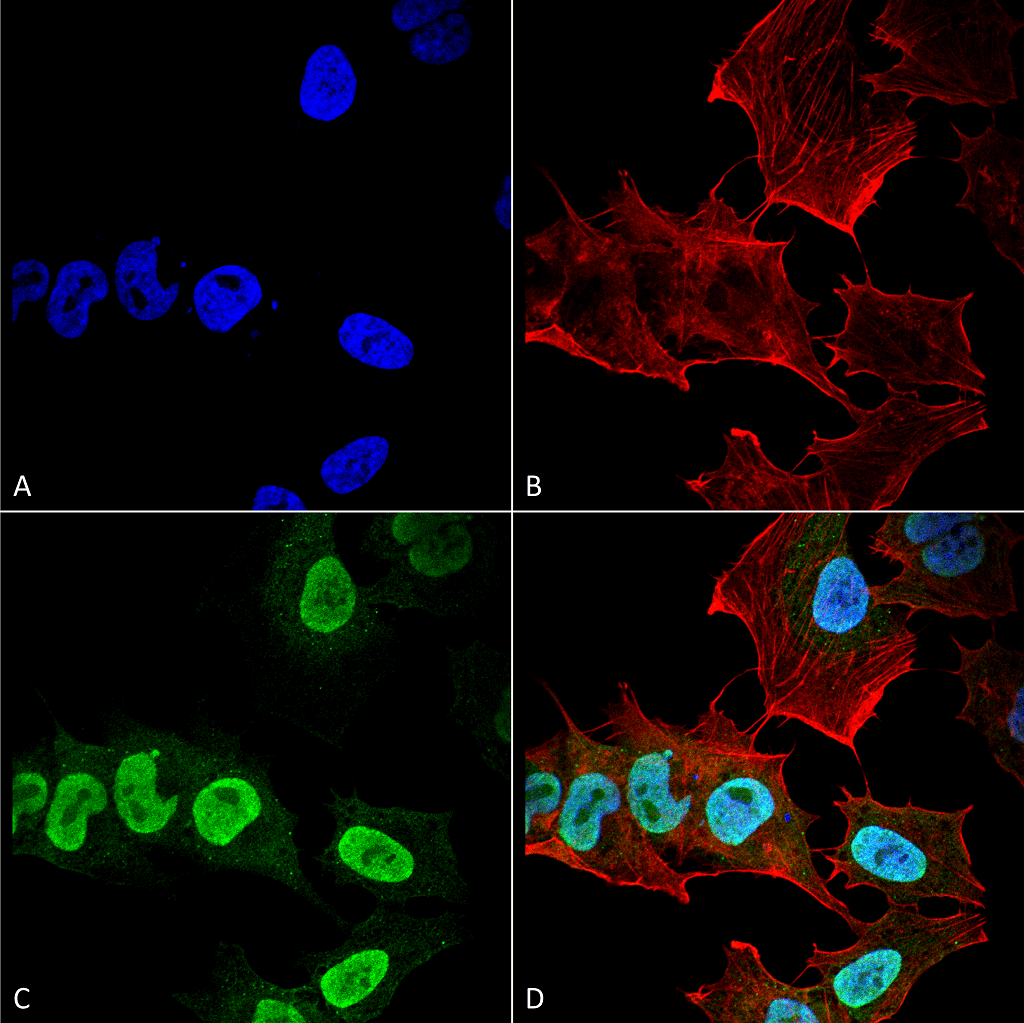
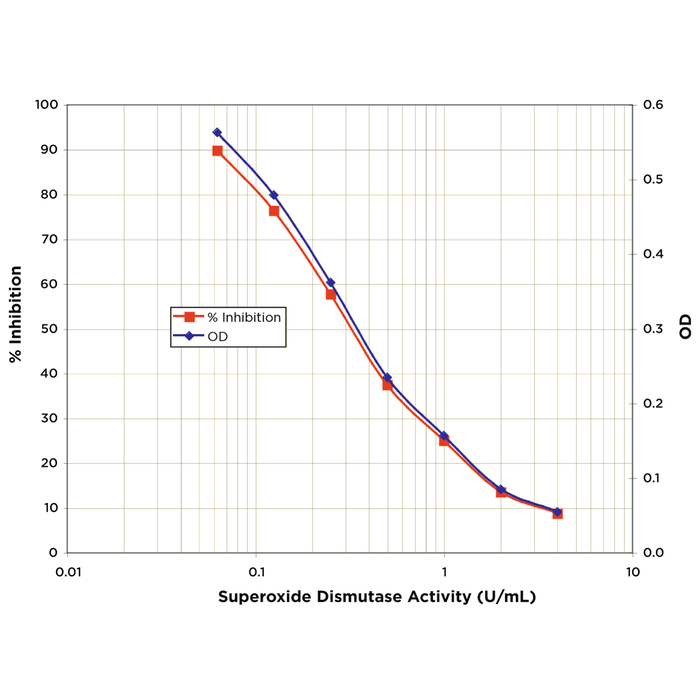
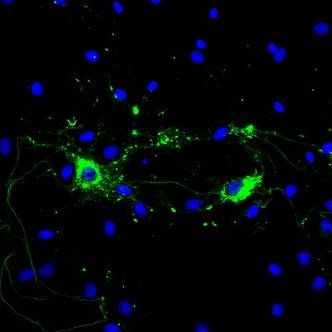
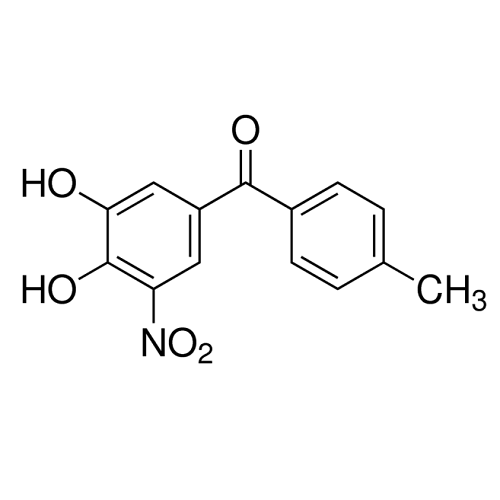
Alpha Synuclein: References
- Goedert, M. (2001). Alpha-Synuclein and Neurodegenerative Diseases. Nature 2: 492 – 501. [PubMed]
- Winner B, Jappelli R, Maji SK, et al. (2011). In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci USA 108: 4194–99. [PubMed]
- Dehay, B., et al. (2015). Targeting α-synuclein for treatment of Parkinson’s disease: mechanistic and therapeutic considerations. The Lancet 14: 855 – 866. [PubMed]
- Pollanen, M.S., Dickson, D.W., and Bergeron, C. (1993). Pathology and Biology of the Lewy Body. Journal of Neuropathology and Experimental Neurology 52 (3): 183 – 191. [PubMed]
- Baba, M., et al. (1998) Aggregation of a-Synuclein in Lewy Bodies of Sporadic Parkinson’s Disease and Dementia with Lewy Bodies. American Journal of Pathology 152(4): 879 -884. [PubMed]
- Hawthorne, G.H., et al. (2016). Nanomedicine to Overcome Current Parkinson’s Treatment Liabilities: A Systematic Review. Neurotox Res 30: 715-729. [PubMed]
- Ariesandi, W., et al. (2013). Temperature-Dependent Structural Changes of Parkinson’s Alpha-Synuclein Reveal the Role of Pre- Existing Oligomers in Alpha-Synuclein Fibrillization. PLOS ONE 8(1): 1-10. [PubMed]
- Roostaee, A.R., Beaudoin, S., Staskeviscius, A., and Roucou, X. (2013). Aggregation and neurotoxicity of recombinant α-synuclein aggregates initiated by dimerization. Molecular Neurodegeneration 8(5): 1-12. [PubMed]
- Volpicelli-Daley, Luk, et al. (2011). Exogenous α-Synuclein Fibrils Induce Lewy Body Pathology Leading to Synaptic Dysfunction and Neuron Death. Neuron 72: 57 – 71. [PubMed]
- Murray, I.V., et al. (2003). Role of α-Synuclein Carboxy-Terminus on Fibril Formation in Vitro. Biochemistry 42: 8530-8540. [PubMed]
- Bendor, J.T., et al. (2013). The Function of α-synuclein. Neuron 79: 1044 -1066. [PubMed]
- Wang W, Perovic I, Chittuluru J, et al. (2011) A soluble α-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci USA 108: 17797–802. [PubMed]
- Lee, H.J., et al. (2011). Enzyme-linked immunosorbent assays for alpha-synuclein with species and multimeric state specificities. Journal of Neuroscience Methods 199: 249-257. [PubMed]
- Theillet, F., et al. (2016). Structural disorder of monomeric [alpha]-synuclein persists in mammalian cells. Nature 530: 1 – 6. [PubMed]
- Yamada, K., and Iwatsubo, T. (2018) Extracellular α-synuclein levels are regulated by neuronal activity. Molecular Neurodegeneration 13(9): 1-8. [PubMed]
- Kahle, P.J., et al. (2002). Structure/function of α‐synuclein in health and disease: rational development of animal models for Parkinson’s and related diseases. Journal of Neurochemistry 82 (3): 449 – 457. [PubMed]
- Sidhu, A., Segers-Nolten, I., Subramaniam V. (2014) Solution conditions define morphological homogeneity of α-synuclein fibrils. Biochimica et Biophysica Acta 1844: 2127-2134. [PubMed]
- Luk, K.C., et al. (2009). Exogenous α-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. PNAS 106 (47): 20051-20056. [PubMed]
- Mahul-Mellier, A-L., et al. (2015). Fibril growth and seeding capacity play key roles in α-synuclein-mediated apoptotic cell death. Cell Death and Differentiation: 1-16. [PubMed]
- Braak, H., et al. (2003). Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging 24: 197 – 211. [PubMed]
- Ubhi, K., Low, P., and Masliah, E. (2011). Multiple system atrophy: a clinical and neuropathological perspective. Trends Neurosci 34: 581–590. [PubMed]
- Konno, M., et al. (2012). Suppression of dynamin GTPase decreases alpha-synuclein uptake by neuronal and oligodendroglial cells: a potent therapeutic target for synucleinopathy. Neurodegener 7:38. DOI: 10.1186/1750-1326-7-38.
- Masuda-Suzukake, M., et al. (2014). Pathological alpha-synuclein propagates through neural networks. Acta Neuropathological Communications 2(88): 1 – 12. [PubMed]
- Bae, E., et al. (2012) Antibody-Aided Clearance of Extracellular α-Synuclein Prevents Cell-to-Cell Aggregate Transmission. The Journal of Neuroscience 32(39): 13454 – 13469. [PubMed]
- Tatenhorst, L., et al. (2016). Fasudil attenuates aggregation of α-synuclein in models of Parkinson’s disease. Acta Neuropathologica Communications 4 (39): 1-17. [PubMed]
- Lee, H., et al. (2002). Formation and Removal of -Synuclein Aggregates in Cells Exposed to Mitochondrial Inhibitors. The Journal of Biological Chemistry 227 (7): 5411-5417. [PubMed]
- Tremlett, H., et al. (2017). The gut microbiome in human neurological disease: A review. Annals of Neurology 81 (3): 369 – 382. [PubMed]
- Bonina F, et al. (2003) Glycosyl derivatives of dopamine and L-dopa as anti-Parkinson prodrugs: synthesis, pharmacological activity and in vitro stability studies. J Drug Target 11:25–36. [PubMed]
- Sharma, S., Lohan, S., and Murthy, R.S.R. Formulation and characterization of intranasal mucoadhesive nanoparticulates and thermo-reversible gel of levodopa for brain delivery. Drug Development and Industrial Pharmacy 40 (7): 869 – 878. [PubMed]
- Paisán-Ruíz, C., et al. (2004). Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron44 (4): 595–600. [PubMed]
- Alessi, D.R. and Sammler, E. (2018). LRRK2 kinase in Parkinson’s disease. Neurodegeneration 360 (6384): 36 – 37. [PubMed]
- Taymans, J.M. and Greggio, E. (2015) LRRK2 kinase inhibition as a therapeutic strategy for Parkinson’s disease, where do we stand? Neuropharmacol. 14: 214–225. [PubMed]
- Witman, G.B., Cleveland, D.W., Weingarten, M.D., and Kirschner, M.W. (1976) Tubulin requires tau for growth onto microtubule initiating sites. Proc Natl Acad Sci USA 73: 4070 –4074. [PubMed]
- Lei, P., et al. (2010). Tau protein: Relevance to Parkinson’s disease. The International Journal of Biochemistry & Cell Biology 42: 1775 – 1778. [PubMed]
- Iba, M., et al. (2013). Synthetic Tau Fibrils Mediate Transmission of Neurofibrillary Tangles in a Transgenic Mouse Model of Alzheimer’s-Like Tauopathy. The Journal of Neuroscience 33(3): 1034-1037. [PubMed]
- Jensen, P.H., et al. (1999) Alpha-synuclein binds to Tau and stimulates the protein kinase A-catalyzed tau phosphorylation of serine residues 262 and 356. The Journal of Biological Chemistry 274: 25481– 9. [PubMed]
- Qureshi, H.Y. and Paudel, H.K. (2011). Parkinsonian Neurotoxin 1-Methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP) and a-Synuclein Mutations Promote Tau Protein Phosphorylation at Ser262 and Destabilize Microtubule Cytoskeleton in Vitro. Journal of Biological Chemistry 286: 5055 – 5068. [PubMed]
- De Vos, K.J., et al. (2008) Role of axonal transport in neurodegenerative diseases. Annual Review of Neuroscience 31:151–73. [PubMed]
- George, J.M. (2002). The synucleins. Genome Biology 3(1): 1-6. [PubMed]
- Galvin, J.E., et al. (1999) Axon pathology in Parkinson’s disease and Lewy body dementia hippocampus contains alpha-, beta-, and gamma-synuclein. Proc Natl Acad Sci USA 96:13450-13455. [PubMed]
- Moriarty, G.M., et al. (2017). A pH-dependent switch promotes β-synuclein fibril formation via glutamate residues. Journal of Biological Chemistry 292(39): 16378-16379. [PubMed]
- Senior, S.L., et al. (2008). Increased striatal dopamine release and hyperdopaminergic‐like behaviour in mice lacking both alpha‐synuclein and gamma‐synuclein. European Journal of Neuroscience 27(4). [PubMed]