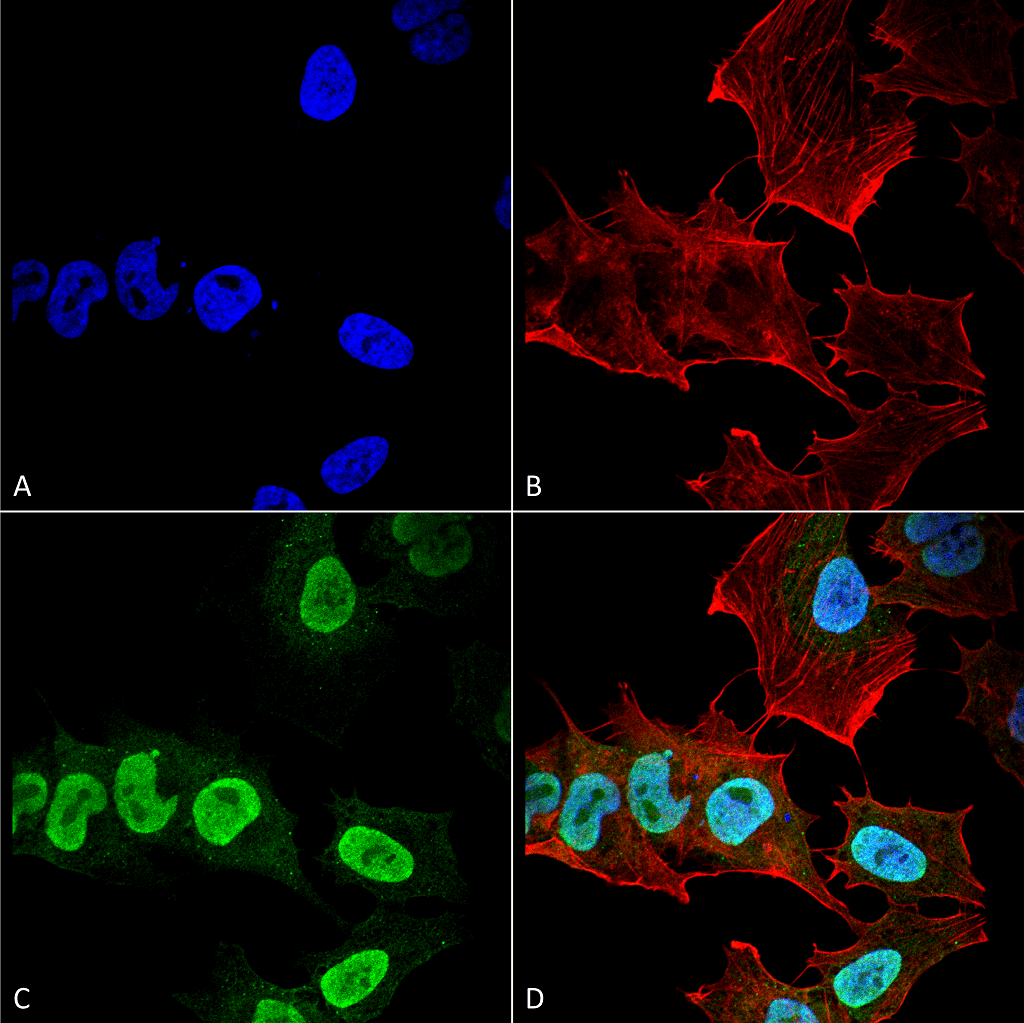
Alpha Synuclein: Aggregation
The characteristic feature of protein misfolding diseases is “the deposition of insoluble protein aggregates and amyloid fibrils caused by accumulation of homogenous proteins which are abnormally folded into β-sheet structures,” (Roostaee et al., 2013). The process of alpha synuclein aggregation starts when monomers accumulate into oligomeric intermediates, followed by the formation of amyloid fibers which cluster into disease specific inclusions (Winner et al., 2011). Roostaee et al. (2013) concluded that a dimeric state exists in the conversion of alpha synuclein monomers to oligomers suggesting that dimerization is the main mechanism initiating protein aggregation.
In the process of alpha synuclein fibrilization, certain solvent conditions can produce specific types of morphology. Elements that promote fibrilization are higher salt concentration and temperature, and an acidic pH17.
Scientists have proposed that either amyloid-like insoluble fibrils or soluble pre-fibrillar intermediates (oligomers and protofibrils) are the toxic species in PD3. Winner et al. (2011) created alpha synuclein mutants that increased either the oligomer or fibril formation in a rat lentivirus system and tested the toxicity by analyzing loss of dopaminergic neurons in the substantia nigra. Their results showed that the alpha synuclein oligomers promote higher levels of toxicity than the alpha synuclein fibrils, making oligomers the toxic species in PD.
Both endogenous and exogenous alpha synuclein participates to induce aggregation and fibrilization. Exogenous alpha synuclein pre-formed fibrils enter the neurons, most likely by absorptive mediated endocytosis, and mediate the recruitment of soluble endogenous alpha synuclein into LBs and LNs9. The exogenous pre-formed alpha synuclein fibrils seed aggregation inside the cell, as monomeric alpha synuclein is not sufficient alone. The alpha synuclein gathered into pathogenic inclusions is phosphorylated at Ser129 and ubiquitinated intracellularly.
Endogenous aggregation is ordinarily limited by the amount of active nucleation sites present in the cytoplasm, however, exogenous α-synuclein solves this dilemma18. The exogenous pre-formed alpha synuclein fibrils act as the “seed” for aggregation by binding to the plasma membrane and becoming nucleation sites for the formation of alpha synuclein fibrils. Binding to the plasma membrane promotes the accumulation and internalization of alpha synuclein aggregates and turns on the extrinsic and intrinsic apoptotic cell death pathways19. In conclusion, LB and LN formation can be initiated by misfolded proteins, and then propagated in the neuronal cells by exogenous alpha synuclein seeding.