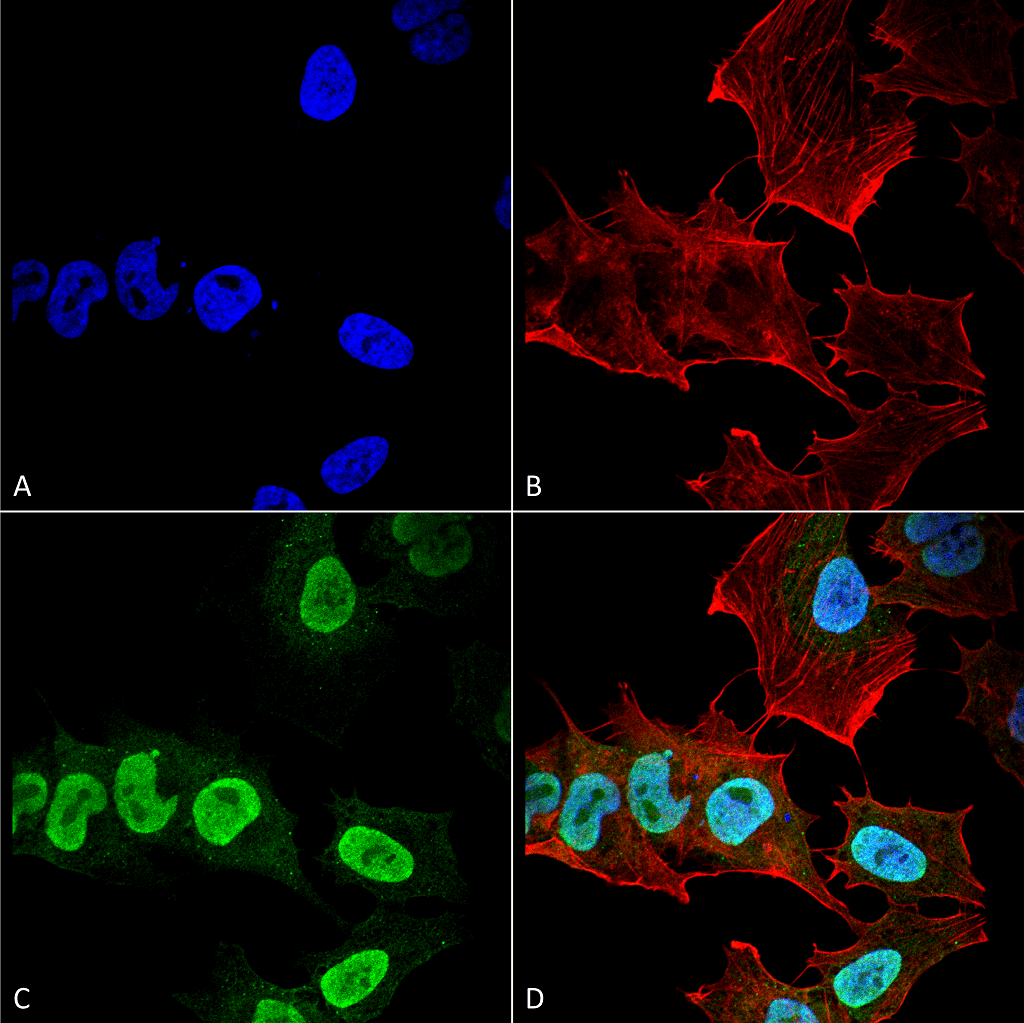
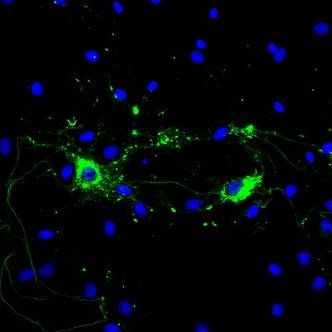
Alpha Synuclein: Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder in the world affecting 1-2 percent of the population over the age of sixty-five1. There are two types of PD: 1) sporadic and 2) familial2. A sporadic or idiopathic disease occurs without genetic cause or family history and makes up the majority of Parkinson’s diagnoses. The familial cases of PD show characteristic point mutations, duplications and triplications of the SCNA gene, which are passed down through generations3. The three defining characteristics of PD are: 1) muscle rigidity, 2) bradykinesia (slowness of movement), and 3) a resting tremor. Additional symptoms include postural instability, cognitive impairment, and festinating gait1.
On a neurological level, PD patients exhibit eosinophilic round cytoplasmic inclusions called Lewy Bodies (LB) and Lewy Neurites (LN)1. LBs and LNs are composed of ubiquitinated neurofilaments and associated proteins that have aggregated in response to cellular stress, genetic mutation, and erroneous phosphorylation4. These hallmark features are formed from degenerating dopaminergic neurons primarily in the substantia nigra of the brain, and in lesser amounts in the hypothalamus and cerebral cortex5. Dopamine is a neurotransmitter that controls movement and enables communication between brain cells, therefore, the loss of dopaminergic neurons results in impaired movement regulation6.
The main component of LB and LN inclusions is alpha synuclein, a soluble, intrinsically disordered protein7 lacking compact secondary and tertiary structures. Alpha synuclein is expressed in the central nervous system (cerebral cortex, hippocampus, amygdala, and olfactory bulb) and concentrated near the presynaptic nerve terminals. Its biological function is unknown; however, many have proposed that alpha synuclein aids in vesicular transport due to its association with vesicular structures8. The accumulation of alpha synuclein, however, causes a decrease in synaptic proteins, gradual impairments in neuronal transmission, and finally, neuronal death9. Due to alpha synuclein’s pathogenic role in the progression of PD, its aggregation has been the target of many therapeutic approaches like antibody mediated clearance and drug induced inhibition. Preventing alpha synuclein accumulation is only one path of PD treatment; LRRK2 kinase, tau, and beta and gamma synuclein are also potential neuroprotective targets in disease therapy.
In summary, PD is a complex disease with many underlying causes, however, understanding its pathogenic mechanism is key in finding potential treatments for those who endure neurological degeneration and suffering.